A Brief Review: Synaptopodin
Synaptopodin
Background
Synaptopodin is an actin-associated protein that may play a role in modulating actin-based shape and motility of dendritic spines and renal podocyte foot processes. It seems to be essential for the formation of spine apparatuses in spines of telencephalic neurons, which is involved in synaptic plasticity. It's gene name is SYNPO. Synaptopodin was first identified in 1991 by Mundel et al (Mundel, Gilbert & Kriz, 1991) as a 44kDa protein related to the actin system of podocyte foot processes.
Cellular Location
The name ‘synaptopodin’ given by Mundel et al (Mundel et al., 1997) reflects the two main known areas of expression: synapto for its expression in brain and podin for its expression kidney podocytes. Podocytes are cells in the Bowman’s capsule of the kidney, wrapping around the capillaries of the glomerulus, forming a filter which allows small molecules through, such as salts and glucose, but acts as a barrier to macromolecules like albumin or globulins. Its presence in the brain appears restricted to neurons in the telencephalon, a complex brain region which incorporating the cerebral cortex, olfactory bulb, hippocampus, basal ganglia and amygdala. It is localized at the tight junction of cells.
Function
We know that synaptopodin is a 929 amino acid protein that is associated with actin, and as such plays some structural role in podocyte cells and telencephalonic neurons, either directly or in regulation. One area synaptopodin is also thought to play a role in is synaptic plasticity – the ability of synapses to strengthen or weaken over time in response to their levels of activity. This is because synaptopodin is found in spine necks of mature dendritic spines, where it is presumed to have a role in defining the actin-based shape of the spine. In other words, in neurons it is thought to mediate changes in the physical shape of the actin cytoskeleton defining the neck of dendritic spines, in response to some plasticity-producing stimulus. The neck of dendritic spines creates a physical barrier which limits how synaptic molecules, including membrane proteins, can diffuse. This suggests that synaptopodin may play a role in regulating this diffusion; widening or closing the ‘net’, as it were. Recent research suggests that synaptopodin may achieve this indirectly via F-actin. (Wang et al., 2016). Functionally speaking, this regulation may play a role in calcium release from the spines. Work by Korkotian et al (Korkotian, Frotscher & Segal, 2014) using hippocampal neurons suggests that synaptopodin positive spines, compared to negative spines enhance calcium release, amplifying the local calcium response and downstream pathways. Inflammation has also been shown to affect synaptopodin expression in the hippocampus. LPS induced inflammation in mice, mimicking systemic inflammation, was shown to reduce synaptopodin expression in the mouse hippocampus as well as affect long-term potentiation (the persistent increase in synapse strength) (Strehl et al., 2014). This suggests that some of the known effects of systemic inflammation on neural plasticity may, to some degree, be due to its effects on synaptopodin expression. Interestingly, neuroinflammation and down-regulation of synaptopodin is seen in Alzheimer’s disease. In kidney, synaptopodin has recently been shown to limit the expression of TRPC6 and attenuate proteinuria (Yu et al., 2016). While over expression of synaptopodin decreased TRCPC6 expression in cultured mouse podocytes, knockout of synaptopodin increased its expression. Interestingly, TRPC6 is a cation (Ca2+) channel receptor, and is also associated with depression and anxiety. While most research has focussed on the role of synaptopodin in podocytes and telenecephalic neurons, other work has found a potential role in endothelial cell wound healing in human umbilical vein endothelial cells. The frictional force on these cells due to the pulsing nature of blood flow (laminar sheer stress) was shown to boost expression of synaptopodin, as well as other actin binding proteins. When synaptopodin expression was suppressed by silencing RNA wound healing was inhibited, whereas overexpression lead to enhanced wound healing (Tang, 2015). Clearly there is still much more to learn about synaptopodin, and its structural and functional roles in podocytes and telenecephalonic neurons.
References
Korkotian, E., Frotscher, M. & Segal, M. (2014) Synaptopodin Regulates Spine Plasticity: Mediation by Calcium Stores. The Journal of Neuroscience. 34 (35), pp. 11641–11651. Mundel, P., Gilbert, P. & Kriz, W. (1991) Podocytes in glomerulus of rat kidney express a characteristic 44 KD protein. [online]. Journal of Histochemistry & Cytochemistry . 39 (8 ), pp. 1047–1056. Mundel, P., Heid, H.W., Mundel, T.M., Krüger, M., Reiser, J. & Kriz, W. (1997) Synaptopodin: An Actin-associated Protein in Telencephalic Dendrites and Renal Podocytes [online]. The Journal of Cell Biology . 139 (1 ), pp. 193–204. Strehl, A., Lenz, M., Itsekson-Hayosh, Z., Becker, D., Chapman, J., Deller, T., Maggio, N. & Vlachos, A. (2014) Systemic inflammation is associated with a reduction in Synaptopodin expression in the mouse hippocampus. Experimental neurology. 261pp. 230–235. Tang, V. (2015) Synaptopodin Couples Epithelial Apical Contractions to Adherens Junction Maturation. The FASEB Journal. 29 (1 Supplement), pp. 1022–1029. Wang, L., Dumoulin, A., Renner, M., Triller, A. & Specht, C.G. (2016) The Role of Synaptopodin in Membrane Protein Diffusion in the Dendritic Spine Neck. PLOS One. pp. 1–18. Yu, H., Kistler, A., Faridi, M.H., Meyer, J.O., Tryniszewska, B., Mehta, D., Yue, L., Dryer, S. & Reiser, J. (2016) Synaptopodin Limits TRPC6 Podocyte Surface Expression and Attenuates Proteinuria. Journal of the American Society of Nephrology. pp. ASN – 2015080896.
ARP Products to Synaptopodin
ARP has a series of highly validated antibodies against Synaptopodin as shown below. We also have a number of ELISA kits targeting Synaptopodin from different species: for the full list click here.
|
03-GP94-IN: Anti-Synaptopodin (IN) |
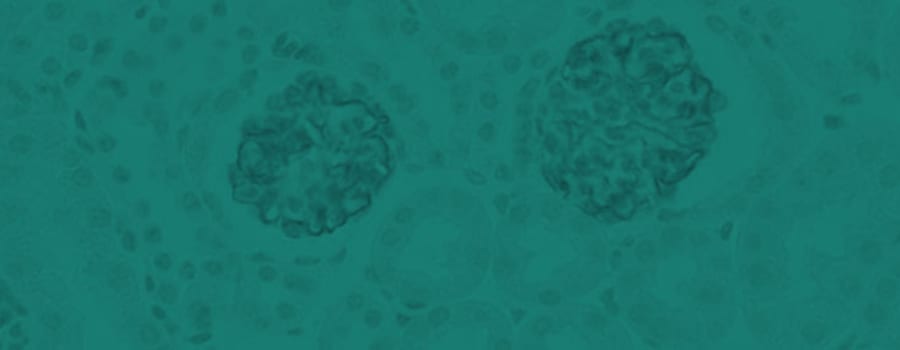
 IHC staining in h. glomeroli (dilution 1:50) IHC staining in h. glomeroli (dilution 1:50) |
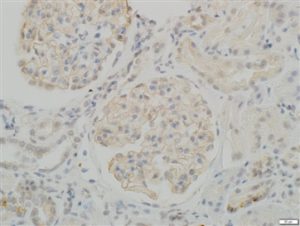
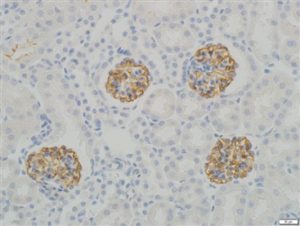 IHC staining in h. glomeroli (dilution 1:100) IHC staining in h. glomeroli (dilution 1:100) |
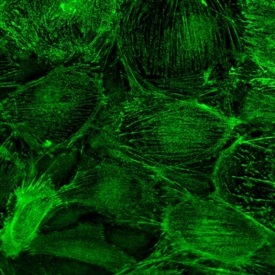 IF staining in h. podocytes IF staining in h. podocytes |
| 03-GP94-INN Anti-Synaptopodin (INN) |  IHC staining in h. glomeroli (dilution 1:100) IHC staining in h. glomeroli (dilution 1:100) |
||
| 03-GP94-C Anti-Synaptopodin, C-Terminal Specific |  IHC staining in h. glomeroli (dilution 1:50) IHC staining in h. glomeroli (dilution 1:50) |
 IHC staining in h. glomeroli (dilution 1:100) IHC staining in h. glomeroli (dilution 1:100) |
 IF staining in h. podocytes IF staining in h. podocytes |
|
03-GP94-N Anti-Synaptopodin, N-Terminal Specific |
 IHC staining in h. glomeroli (dilution 1:50) IHC staining in h. glomeroli (dilution 1:50) |
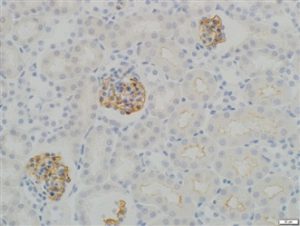 IHC staining in h. glomeroli (dilution 1:100) IHC staining in h. glomeroli (dilution 1:100) |
 IF staining in h. podocytes IF staining in h. podocytes |
Download PDF :  Filed Under : Antibodies, Product Review
Filed Under : Antibodies, Product Review


